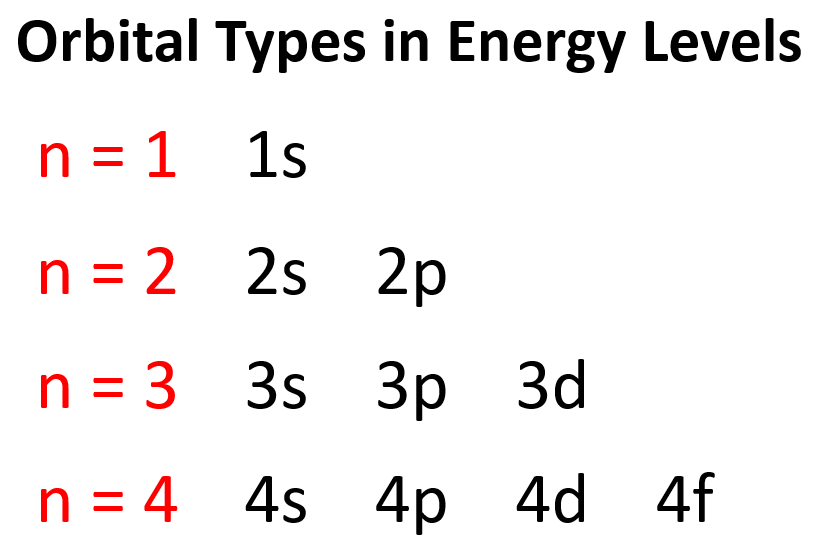
Quantum Mechanical Energy Level Definition . Generally speaking, the energy of an electron in an. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). An atom (the nucleus and the. in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in quantum mechanics, the energy of a confined system can take on only certain quantized values. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. the energy levels are labeled with an n value, where n = 1, 2, 3,.
from general.chemistrysteps.com
Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). in quantum mechanics, the energy of a confined system can take on only certain quantized values. An atom (the nucleus and the. in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. Generally speaking, the energy of an electron in an. the energy levels are labeled with an n value, where n = 1, 2, 3,.
s, p, d, f Atomic Orbitals Chemistry Steps
Quantum Mechanical Energy Level Definition in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. An atom (the nucleus and the. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). Generally speaking, the energy of an electron in an. the energy levels are labeled with an n value, where n = 1, 2, 3,. in quantum mechanics, the energy of a confined system can take on only certain quantized values. in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe.
From www.slideserve.com
PPT The Quantum Model PowerPoint Presentation, free download ID2574252 Quantum Mechanical Energy Level Definition the energy levels are labeled with an n value, where n = 1, 2, 3,. An atom (the nucleus and the. Generally speaking, the energy of an electron in an. in quantum mechanics, the energy of a confined system can take on only certain quantized values. in the limit of large energies, the predictions of quantum mechanics. Quantum Mechanical Energy Level Definition.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Quantum Mechanical Energy Level Definition atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation ID Quantum Mechanical Energy Level Definition the energy levels are labeled with an n value, where n = 1, 2, 3,. An atom (the nucleus and the. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in quantum mechanics, the energy of a confined system can take on only certain quantized values. atoms. Quantum Mechanical Energy Level Definition.
From www.researchgate.net
a) Energy level structure of a double quantum dot with levels εL and Quantum Mechanical Energy Level Definition in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. Generally speaking, the energy of an electron in an. An atom (the nucleus and the. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). in fact,. Quantum Mechanical Energy Level Definition.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 Quantum Mechanical Energy Level Definition Generally speaking, the energy of an electron in an. An atom (the nucleus and the. in quantum mechanics, the energy of a confined system can take on only certain quantized values. the energy levels are labeled with an n value, where n = 1, 2, 3,. in fact, quantum mechanics exactly predicts the energy shells and the. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Unit 3 Atomic Theory & Quantum Mechanics Section A.6 A.7 Quantum Mechanical Energy Level Definition in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. in quantum mechanics, the energy of a confined system can take on only certain quantized values. the energy levels are labeled with an n value, where n = 1, 2, 3,. Quantum energy levels are discrete values of energy that an. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation Quantum Mechanical Energy Level Definition in quantum mechanics, the energy of a confined system can take on only certain quantized values. the energy levels are labeled with an n value, where n = 1, 2, 3,. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). in fact, quantum. Quantum Mechanical Energy Level Definition.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Quantum Mechanical Energy Level Definition the energy levels are labeled with an n value, where n = 1, 2, 3,. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in quantum mechanics, the energy of a confined system can take on only certain quantized values. in fact, quantum mechanics exactly predicts the. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT The Quantum Mechanical Model of the Atom PowerPoint Presentation Quantum Mechanical Energy Level Definition the energy levels are labeled with an n value, where n = 1, 2, 3,. Generally speaking, the energy of an electron in an. in quantum mechanics, the energy of a confined system can take on only certain quantized values. An atom (the nucleus and the. Quantum energy levels are discrete values of energy that an electron in. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT The Quantum Mechanical Model of the Atom PowerPoint Presentation Quantum Mechanical Energy Level Definition Generally speaking, the energy of an electron in an. An atom (the nucleus and the. the energy levels are labeled with an n value, where n = 1, 2, 3,. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. atoms have a series of energy levels called principal energy levels,. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation Quantum Mechanical Energy Level Definition Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. the energy levels are labeled with an n value, where n = 1, 2, 3,. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. Generally speaking, the energy of an electron in. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Chapter 4 Electron Structure of the Atom PowerPoint Presentation Quantum Mechanical Energy Level Definition An atom (the nucleus and the. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. the energy levels are labeled with an n value, where n = 1, 2, 3,. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1,. Quantum Mechanical Energy Level Definition.
From mungfali.com
Electron Energy Level 8C2 Quantum Mechanical Energy Level Definition in quantum mechanics, the energy of a confined system can take on only certain quantized values. Generally speaking, the energy of an electron in an. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum. Quantum Mechanical Energy Level Definition.
From www.britannica.com
Physics Quantum Mechanics, Particles, Waves Britannica Quantum Mechanical Energy Level Definition in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. An atom (the nucleus and the. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical. Quantum Mechanical Energy Level Definition.
From drivenheisenberg.blogspot.com
The Figure Is An Energy Level Diagram For A Quantum Systemfigure 1 Quantum Mechanical Energy Level Definition in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. Generally. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download Quantum Mechanical Energy Level Definition Generally speaking, the energy of an electron in an. in quantum mechanics, the energy of a confined system can take on only certain quantized values. the energy levels are labeled with an n value, where n = 1, 2, 3,. An atom (the nucleus and the. in fact, quantum mechanics exactly predicts the energy shells and the. Quantum Mechanical Energy Level Definition.
From www.youtube.com
Understanding Quantum Mechanics 7 Atomic Energy Levels YouTube Quantum Mechanical Energy Level Definition An atom (the nucleus and the. Quantum energy levels are discrete values of energy that an electron in an atom can possess, determined by the. in the limit of large energies, the predictions of quantum mechanics agree with the predictions of classical mechanics. in quantum mechanics, the energy of a confined system can take on only certain quantized. Quantum Mechanical Energy Level Definition.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download Quantum Mechanical Energy Level Definition atoms have a series of energy levels called principal energy levels, which are designated by whole numbers (n = 1, 2, 3,.). Generally speaking, the energy of an electron in an. in fact, quantum mechanics exactly predicts the energy shells and the hydrogen atom spectrum we observe. in quantum mechanics, the energy of a confined system can. Quantum Mechanical Energy Level Definition.